JSR Medical holds the world’s best minimally invasive colorectal surgical technology.
We develop innovative surgical devices and treatment methods that minimize patient’s stress and promote fast recovery.
Our goal is to enhance patient’s quality of life by developing revolutionary surgical devices and technology.

- March 2016 Establishment of JSR Medical Co., Ltd.
- April 2016 Medical Device Sales Registration (MFDS 2016-3430019-00008)
- August 2018 DGIST Authentication (Industry-University Cooperation 2018-031)
- November 2018 Frontier Venture Certification (0068)
- May 2020 Medical Device Manufacturer Registration (MFDS 6855)
- July 2020 Medical Device Manufacturing & Export Registration (MFDS 20-550)
- November 2020 Excellent Technology Company Certification (FTS-2020-03072) / Venture Company Certification (20200116111)
- December 2020 Manufacturing Site Registration (272902020549805)
- January 2021 ISO 13485:2016 Approval (GIS-5073-MD)
- June 2021 ‘COLO-BT™’ Brand Registration (40-1741587 and 1 other)
- November 2021 Corporate R&D Center Registration (2021115800)
- January 2022 KGMP Approval (KCL-AABA-13323)
- July 2022 EN ISO 13485:2016 Approval (Medical Device Class IIa)
- August 2022 FDA Registration (Medical Device Class I)
- December 2022 MDSAP Approval (MDSAP 757220)
- January 2023 FDA IDE Approval (G220334 – Medical Device Class III)
- February 2023 MOU with K-MEDI hub Preclinical Center Signed
- April 2023 Initiation of COLO-BT™ Multicenter-Pivotal Clinical Trial at State University of New York
- December 2023 First-patient-in (University at Buffalo)
- April 2024 Exhibitor at the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES)
- June 2024 Exhibitor at the American Society of Colon and Rectal Surgeons (ASCRS) and selected for a Break-through Technology
- July 2024 Initiation of COLO-BT™ Multicenter-Pivotal Clinical Trial at Penn State Health Medical Center
- October 2024 Sponsor for the Asian Robotic Camp for Colorectal Surgeons (ARCCS)
- December 2024 Series A funding for KRW 5.5 billion (approx. USD 4 million)
- March 2025 Exhibitor at the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES)
- April 2025 Exhibitor at the Korean Society of Coloproctology (KSCP)
- May 2025 Exhibitor at the American Society of Colon and Rectal Surgeons (ASCRS)
- August 2025 Exhibitor at the Korean Colorectal Surgeon Southern Study Group (K-CROSS) / Exhibitor at the International Colorectal Research Summit (iCRS)
PRODUCT
COLO-BT™
COLO-BT™ is a stool bypass device that replaces the conventional temporary ileostomy and eliminates the need for an ostomy bag and the reversal surgery. It has a unique irrigation function that allows patients to manage their bowels without the need for laxatives.
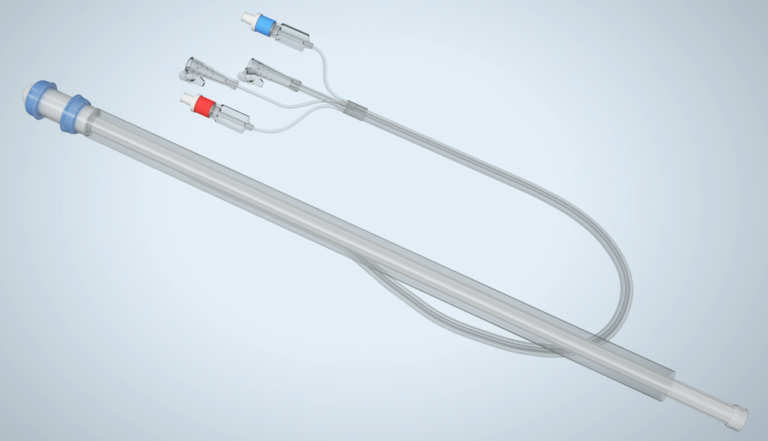

Development Background
However, there are various complications associated with a stoma, and the occurrence rate of these complications is 25 – 50%. Furthermore, 12 – 40% of patients cannot have stoma reversal surgery and must wear an ileostomy bag for the rest of their lives.
JSR has invented an innovative medical device that minimizes complications and eliminates the need for a stoma, which significantly improves the quality of a patient’s life.

Indication for Use
- Alternative treatment device to the temporary ileostomy following colorectal surgery.
- Protects the patient's skin and anastomotic wound to prevent complications from anastomotic leakage and to provide access for colonic irrigation.
PRODUCT
Drain-TG™
Drain-TG™ is an Anastomotic Leakage Monitoring and Drainage (ALMOND) system, utilizing the extra-peritoneal tunneling method. It allows early detection of anastomotic leakage and immediate treatment without surgical re-intervention.


Development Background
Therefore, drain tubes have to be placed before anastomotic leakage to prevent complications.
The conventional way of inserting the drain tube is using laparoscopic instruments, and there is a problem that it is not fixed and may migrate.
JSR has invented a new drainage system that uses the extra-peritoneal tunneling method to guide the drain tube to be inserted and fixed at the anastomotic site to minimize complications.

Indication for Use
- A single-use device for immediate diagnosis and treatment of anastomotic leakage at the anastomotic site.
- Use the extra-peritoneal tunneling method to fix the drain tube at the anastomotic site.





